Headed by Prof. Galina A. Tsirlina (e-mail: tsir@elch.chem.msu.ru)
Elctrocrystallization of oxides MOn result from electrochemical transformations of the soluble M compounds with formation of less soluble solid products at the electrode surface. In contrast to alternative processes of oxides electrodeposition induced by pH increase near the electrode and not accompanied by any M electrochemical reactions, MOn electrocrystallization is directly controlled by electrode potential. It demonstrates a number of similarities to well-studied metal electrocrystallization processes, but more complex. In particular, the induction period before oversaturation is typically much longer, and the charge spent for this period is essential. Various chemical steps are unavoidable in the course of oxides electrocrystallization, including bond rupture steps. Moreover, the oxygen stoichiometry of the resulting solids can be potential-dependent.
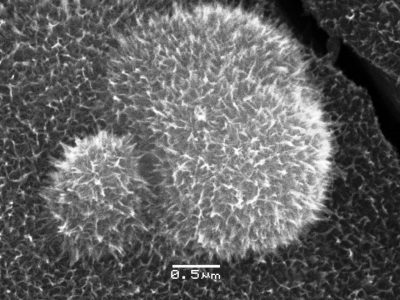
This is electrodeposited birnessite
We are dealing with both cathodic and anodic oxides electrocrystallization. In the context of material science our electrodeposition research is of interest for fabrication of materials which later operate in the same or similar media, with much weaker manifestations of degradation as compared to materials fabricated under ‘dry’ conditions and degrading in solutions. This is the reason why we accent the materials for electrochemcal devices (electrochromic, rechargeable, and electrocatalytic).
- L.V. Pugolovkin, O.V. Cherstiouk, L.M. Plyasova, I.Y. Molina, T.Y. Kardash, O.A. Stonkus, D.A. Yatsenko, V.V. Kaichev, G.A. Tsirlina. Electrodeposited non-stoichiometric tungstic acid for electrochromic applications: film growth modes, crystal structure, redox behavior and stability. Appl. Surface Sci., 2016. 388: p. 786-793. DOI: 10.1016/j.apsusc.2016.04.168.
- A.S. Ryabova, A. Bonnefont, P. Zagrebin, T. Poux, R. Paria Sena, J. Hadermann, A.M. Abakumov, G. Kéranguéven, S.Y. Istomin, E.V. Antipov, G.A. Tsirlina, E.R. Savinova. Study of Hydrogen Peroxide Reactions on Manganese Oxides as a Tool To Decode the Oxygen Reduction Reaction Mechanism. ChemElectroChem, 2016. 3(10): p. 1667-1677. DOI: 10.1002/celc.201600236.
- A.S. Ryabova, A. Bonnefont, P.A. Simonov, T. Dintzer, C. Ulhaq-Bouillet, Y.G. Bogdanova, G.A. Tsirlina, E.R. Savinova. Further insights into the role of carbon in manganese oxide/carbon composites in the oxygen reduction reaction in alkaline media. Electrochim. Acta, 2017. 246: p. 643-653. DOI: 10.1016/j.electacta.2017.06.017.