The research in this direction is aimed at combining molecular modeling of the reactant geometrical and electronic properties, the energetics of solvation and solvent reorganization, reaction layer structure, etc., and the experimental verification of the theoretical predictions. The range of model reactants includes transition metal complexes and simple organic molecules (Cr[EDTA]–, Co[EDTA]–, metallocenes, substituted quinones). The choice of simple model reactants enables accurate electron transfer (ET) rates determination, which allows for the analysis of correlations between the experimental values of heterogeneous rate constants and solvent dynamics, reorganization energies and other “microscopic” parameters of the theory. We use quantum chemical and molecular dynamics methods to explore solvation shell structures, work terms and electronic transmission coefficient distance dependences:
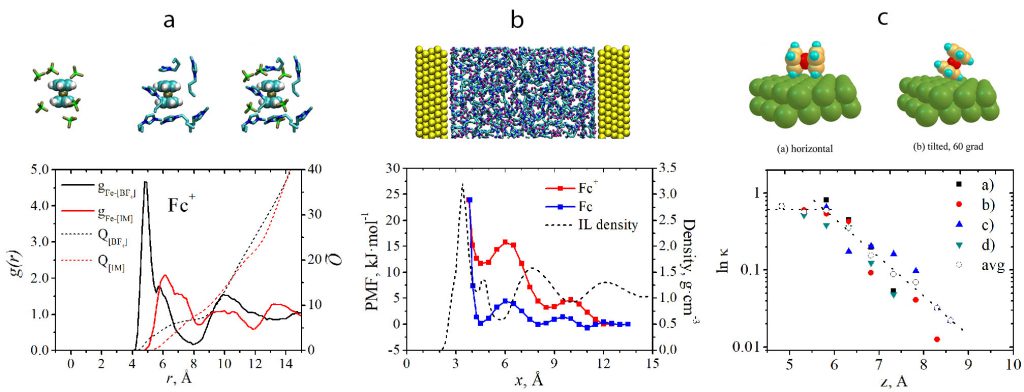
(a) Radial distribution functions for Fe atom in Fc+ and BF4– and [С4mim]+ centers of mass; (b) potentials of mean force for Fc and Fc+ in the vicinity of Au(111) electrode surface; (c) distance dependence of electronic transmission coefficient. (V.A. Nikitina et al., J. Phys. Chem. C. 118 (2014) 6151).
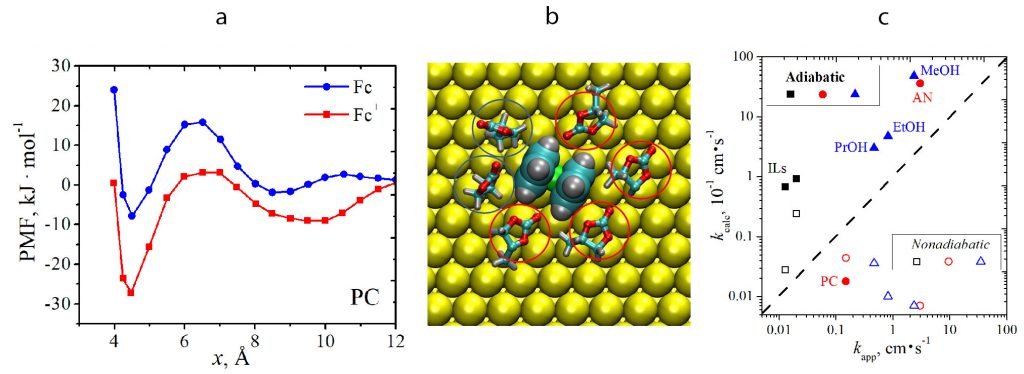
Construction of the molecular level picture of the ET elementary act (with the account for the dynamic and static solvent effects, reactant’s distance of closest approach, electron transfer probability) can be used to diagnose the ET mechanism and to specify the critical rate-controlling factors. This kind of analysis is more straightforward for simple one-electron processes (e.g. Fc+/Fc reaction). For instance, it can be deduced that specific adsorption of Fc+ in propylene carbonate causes an increase in the distance of closest approach, which results in a substantial decrease in the ET rate and drives the reaction from adiabatic to nonadiabatic regime:
(a) Potentials of mean force for Fc and Fc+ in the vicinity of Au(111) in propylene carbonate; (b) adsorbed Fc+; (с) experimental and computed for adiabatic and nonadiabatic regimes ET rate constants for Fc+/Fc process in different solvents.