Multistep electrocatalytic reactions demonstrate the most complex kinetic patterns (e.g. oxygen reduction and oxygen evolution reactions). In this case, the research includes modeling of solid catalytic surfaces and reaction intermediates in the adsorbed state, as well as computing activation energies and preexponential factors for charge transfer and bond-dissociation/formation events. The additional difficulty consists in establishing the nature of the reaction limiting step and mechanisms of consecutive steps (outersphere or innersphere reactions).
Our research is focused on the computational description of the oxygen reduction reaction kinetics at the surfaces of transition metal oxides (Mn oxides). Modeling of the catalytic surfaces and estimation of activation barriers is performed using DFT methods.
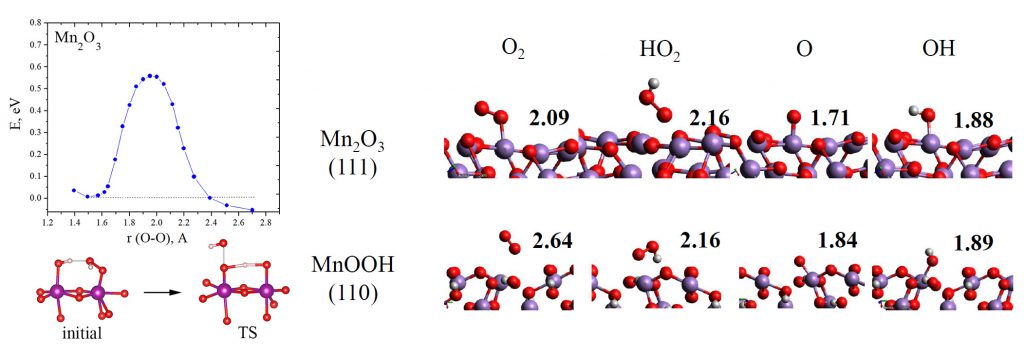
Activation barrier for O-O bond breaking in adsorbed HO2– intermediate at the surface of Mn2O3 oxide; geometries of the adsorbed intermediates of ORR at Mn oxides.
References
- V.A. Nikitina, A.A. Kurilovich, A. Bonnefont, A.S. Ryabova, R.R. Nazmutdinov, E.R. Savinova, G.A. Tsirlina. ORR on Simple Manganese Oxides: Molecular-Level Factors Determining Reaction Mechanisms and Electrocatalytic Activity. J. Electrochem. Soc., 165:15 (2018) J3199-J3208.