Vladimir Sergeevich Bagotsky
(22.01.1920 – 12.11.2012)

![]() Vladimir Sergeevich Bagotsky (22.01.1920, Bern, Switzerland – 12.11.2012, Colorado, USA) was one of the pioneers of modern electrochemistry, and was justly famous for his penetrating analyses of electrochemical problems. Among his friends and colleagues, and certainly for the authors of this introductory text, he is remembered as a brilliant teacher and trusted friend, and as someone who was at ease with people of all ages. Among the wider electrochemical community, he is perhaps best known as the author of a fine textbook ([7, 8] in the list of Monographs).
Vladimir Sergeevich Bagotsky (22.01.1920, Bern, Switzerland – 12.11.2012, Colorado, USA) was one of the pioneers of modern electrochemistry, and was justly famous for his penetrating analyses of electrochemical problems. Among his friends and colleagues, and certainly for the authors of this introductory text, he is remembered as a brilliant teacher and trusted friend, and as someone who was at ease with people of all ages. Among the wider electrochemical community, he is perhaps best known as the author of a fine textbook ([7, 8] in the list of Monographs).
![]() Vladimir Sergeevich Bagotsky was born in Switzerland, the son of Sergey Yustinovich Bagotsky and Regina Eduardovna Birenbaum. Before the First World War, his father had been an activist of the Russian social-democratic labor party, who moved to Switzerland in 1914. After diplomatic relations between Russia and Switzerland were severed in 1918, he became the Soviet Red Cross representative in Geneva, and it was through those offices that political contact was maintained between the two states until 1936. In 1938, after the teenage Vladimir had completed his secondary education in Switzerland, the family returned to Russia, and the young man entered the Chemical Faculty of Moscow State University (MSU).
Vladimir Sergeevich Bagotsky was born in Switzerland, the son of Sergey Yustinovich Bagotsky and Regina Eduardovna Birenbaum. Before the First World War, his father had been an activist of the Russian social-democratic labor party, who moved to Switzerland in 1914. After diplomatic relations between Russia and Switzerland were severed in 1918, he became the Soviet Red Cross representative in Geneva, and it was through those offices that political contact was maintained between the two states until 1936. In 1938, after the teenage Vladimir had completed his secondary education in Switzerland, the family returned to Russia, and the young man entered the Chemical Faculty of Moscow State University (MSU).
 |
| Graduates of gymnasium in Bern in 1938 and 50 years later. Vladimir Bagotsky is the first from the left in the first row (upper photo) and the third from the left, sitting (lower photo) |
 |
| Vladimir Bagotsky during his gymnasium period in Bern (1930s) |
![]() During his student years (1938-1944), which coincided with the Second World War, Bagotsky developed his life-long interest in electrochemistry. His initial studies (on hydrogen evolution) were inspired by Alexander Naumovich Frumkin (1895-1976), who had become the head of the Laboratory of Engineering Electrochemistry at Moscow State University in 1930, and the head of the newly founded Department of Electrochemistry in 1933. Frumkin had developed a world-leading school devoted to the study of electrode kinetics and the structure of the double layer. These fields were strongly interrelated, of course, and had led to the discovery of the “Frumkin correction” (initially known as the ‘psi-prime effect’).
During his student years (1938-1944), which coincided with the Second World War, Bagotsky developed his life-long interest in electrochemistry. His initial studies (on hydrogen evolution) were inspired by Alexander Naumovich Frumkin (1895-1976), who had become the head of the Laboratory of Engineering Electrochemistry at Moscow State University in 1930, and the head of the newly founded Department of Electrochemistry in 1933. Frumkin had developed a world-leading school devoted to the study of electrode kinetics and the structure of the double layer. These fields were strongly interrelated, of course, and had led to the discovery of the “Frumkin correction” (initially known as the ‘psi-prime effect’).
![]() Bagotsky initially began work with Zinoviy Alexandrovich Iofa [Jofa] (1895-1989), who was one of Frumkin’s closest collaborators. At the time, the evolution of hydrogen on mercury was a subject of great international curiosity, and Bagotsky set to work on it [2]. After studying this reaction for a while, Bagotsky (together with Frumkin and Iofa) found himself in a dispute with Nikolay Ivanovich Kobozev (1903-1974), who had speculated that the reaction intermediate is atomic hydrogen dissolved in water. Kobozev was a prominent physical chemist and founder of the Laboratory of Catalysis and Gas Electrochemistry at MSU, and it took considerable effort on Bagotsky’s part [1, 3] to resolve the issue in frames of Frumkin’s slow discharge theory. The final arguments are summarized in Refs [7, 9].
Bagotsky initially began work with Zinoviy Alexandrovich Iofa [Jofa] (1895-1989), who was one of Frumkin’s closest collaborators. At the time, the evolution of hydrogen on mercury was a subject of great international curiosity, and Bagotsky set to work on it [2]. After studying this reaction for a while, Bagotsky (together with Frumkin and Iofa) found himself in a dispute with Nikolay Ivanovich Kobozev (1903-1974), who had speculated that the reaction intermediate is atomic hydrogen dissolved in water. Kobozev was a prominent physical chemist and founder of the Laboratory of Catalysis and Gas Electrochemistry at MSU, and it took considerable effort on Bagotsky’s part [1, 3] to resolve the issue in frames of Frumkin’s slow discharge theory. The final arguments are summarized in Refs [7, 9].
![]() Before the development of the rotating disk electrode by Levich and others, the main apparatus for performing electrochemical kinetic studies was the dropping mercury electrode. An unsolved problem was the precise relation between the expanding interface and the observed current. At the time, experimental data were typically interpreted in terms of “activation overpotential” or “concentration polarisation”, or sometimes mixtures of both. These concepts had evolved over a long time, in parallel with the development of galvanostatic instrumentation, and correspond (roughly speaking) to modern “Tafel behaviour” and “diffusion control”. In the case of the dropping mercury electrode, the correction for concentration polarization required the solution of a difficult moving boundary problem. Certain mathematical solutions were first derived by Naum Natanovich Meiman (1911-2001), and then the results were successfully applied to the dropping mercury electrode by Bagotsky [4]. To this day, the analysis of polarographic data in the mixed kinetic regime is known as the Maiman-Bagotsky technique.
Before the development of the rotating disk electrode by Levich and others, the main apparatus for performing electrochemical kinetic studies was the dropping mercury electrode. An unsolved problem was the precise relation between the expanding interface and the observed current. At the time, experimental data were typically interpreted in terms of “activation overpotential” or “concentration polarisation”, or sometimes mixtures of both. These concepts had evolved over a long time, in parallel with the development of galvanostatic instrumentation, and correspond (roughly speaking) to modern “Tafel behaviour” and “diffusion control”. In the case of the dropping mercury electrode, the correction for concentration polarization required the solution of a difficult moving boundary problem. Certain mathematical solutions were first derived by Naum Natanovich Meiman (1911-2001), and then the results were successfully applied to the dropping mercury electrode by Bagotsky [4]. To this day, the analysis of polarographic data in the mixed kinetic regime is known as the Maiman-Bagotsky technique.
 |
| Alexander Naumovich Frumkin and his collaborators in MSU in the 1940s. Sitting, first row (from left to right): Mikhail Abramovich Gerovich, Alexander Naumovich Frumkin, Zinoviy Alexandrovich Iofa, Amaliya Davydovna Obrucheva. Second row: Vasily Alexandrovich Kuznetsov, Boris Stepanovich Gurenkov, Vladimir Sergeevich Bagotsky, two unknown ladies, Natalya Borisovna Moisseeva, Anna Ivanovna Fedorova, Sofya Yakovlena Mirlina |
 |
| Zinoviy Alexandrovich Iofa (left) and Boris Nikolaevich Kabanov (right), who co-authored the textbook “Kinetics of electrode processes” with A.N. Frumkin and V.S. Bagotsky |
![]() During his student years (1938-1944), which After the award of his PhD (1947), Bagotsky initially remained at MSU. He supervised several diploma and doctoral students, and made important records of Frumkin’s lectures (which were later transformed into the book ‘Kinetics of Electrode Processes’, authored by A. N. Frumkin, V. S. Bagotsky, Z. A. Iofa, and B. N. Kabanov. (See [1] in Monographs subsection). He also began work on the oxygen reduction reaction. However, in 1949, following an outburst of state-sponsored anti-Semitism (named euphemistically as “the struggle against rootless cosmopolitans”) Vladimir Sergeevich was forced to leave his alma mater. After many fruitless attempts to find a job he was finally hired by the All-Union Research Institute “Cells-Electrocarbon”, which had been founded to develop power sources for the radio industry. Later this Institute was renamed the All-Union Institute of Power Sources (abbreviated in Russian as VNIIT). Eventually, Bagotsky was able to publish some of his MSU work, such as that [8, 10-12] performed with Irina Evgenyevna Yablokova (1923-2014), who had become his second wife. Irina had started to work with Vladimir as a diploma student in 1947, and they continued to work together throughout their long professional life. Later, after retirement and moving to the USA, they wrote a joint memoir, but only a few copies were printed and distributed in their closest surrounding.
During his student years (1938-1944), which After the award of his PhD (1947), Bagotsky initially remained at MSU. He supervised several diploma and doctoral students, and made important records of Frumkin’s lectures (which were later transformed into the book ‘Kinetics of Electrode Processes’, authored by A. N. Frumkin, V. S. Bagotsky, Z. A. Iofa, and B. N. Kabanov. (See [1] in Monographs subsection). He also began work on the oxygen reduction reaction. However, in 1949, following an outburst of state-sponsored anti-Semitism (named euphemistically as “the struggle against rootless cosmopolitans”) Vladimir Sergeevich was forced to leave his alma mater. After many fruitless attempts to find a job he was finally hired by the All-Union Research Institute “Cells-Electrocarbon”, which had been founded to develop power sources for the radio industry. Later this Institute was renamed the All-Union Institute of Power Sources (abbreviated in Russian as VNIIT). Eventually, Bagotsky was able to publish some of his MSU work, such as that [8, 10-12] performed with Irina Evgenyevna Yablokova (1923-2014), who had become his second wife. Irina had started to work with Vladimir as a diploma student in 1947, and they continued to work together throughout their long professional life. Later, after retirement and moving to the USA, they wrote a joint memoir, but only a few copies were printed and distributed in their closest surrounding.
 |
| Vladimir Bagotsky and Irina Yablokova: MSU period, VNIITIELAN period, and USA period |
![]() The drastic change in research style, from fundamental theory at MSU to applied engineering at VNIIT, was inevitably accompanied by a decrease in published output. However, there was no decrease in creativity. On the contrary, Bagotsky contributed substantially to the development of a series of innovative batteries for submarines, aircraft, and spacecraft, most notably silver-zinc batteries, mercury-zinc batteries, water-activated batteries, and thermal reserve batteries. The first man-made space satellite “Sputnik”, which was launched on October 4, 1957, was equipped with three silver-zinc batteries made under Bagotsky’s supervision. Later, other Soviet spacecraft (including the mighty “Vostok” with Yuri Gagarin in 1961) were equipped with these batteries. On account of these achievements, Bagotsky was awarded the degree of the Doctor of Technical Sciences (honoris causa) without presenting a thesis, in 1959. By this time he had twice been awarded the Order of the Red Banner (1956 and 1957) and in 1961 he was finally awarded the Order of Lenin, the highest decoration bestowed by the Soviet Union.
The drastic change in research style, from fundamental theory at MSU to applied engineering at VNIIT, was inevitably accompanied by a decrease in published output. However, there was no decrease in creativity. On the contrary, Bagotsky contributed substantially to the development of a series of innovative batteries for submarines, aircraft, and spacecraft, most notably silver-zinc batteries, mercury-zinc batteries, water-activated batteries, and thermal reserve batteries. The first man-made space satellite “Sputnik”, which was launched on October 4, 1957, was equipped with three silver-zinc batteries made under Bagotsky’s supervision. Later, other Soviet spacecraft (including the mighty “Vostok” with Yuri Gagarin in 1961) were equipped with these batteries. On account of these achievements, Bagotsky was awarded the degree of the Doctor of Technical Sciences (honoris causa) without presenting a thesis, in 1959. By this time he had twice been awarded the Order of the Red Banner (1956 and 1957) and in 1961 he was finally awarded the Order of Lenin, the highest decoration bestowed by the Soviet Union.
![]() Although much of his industrial work was carried out in secrecy, Bagotsky nevertheless managed to publish a small amount of fundamental research concerning the fundamentals of power sources. Together with his colleagues in VNIIT, and with collaborators at other research centers (most notably Moscow University and the Institute of Physical Chemistry) Bagotsky published studies on chromic acid reduction at carbon electrodes. This work was written jointly with Galina Vladimirovna Shteinberg [13] in 1957. Work was also published on the kinetics of mercury-zinc cells (jointly with Emil’ Alexandrovich Mendgeritsky) [14, 22], and on the kinetics of zinc electrodes in alkaline solutions [16-21, 26].
Although much of his industrial work was carried out in secrecy, Bagotsky nevertheless managed to publish a small amount of fundamental research concerning the fundamentals of power sources. Together with his colleagues in VNIIT, and with collaborators at other research centers (most notably Moscow University and the Institute of Physical Chemistry) Bagotsky published studies on chromic acid reduction at carbon electrodes. This work was written jointly with Galina Vladimirovna Shteinberg [13] in 1957. Work was also published on the kinetics of mercury-zinc cells (jointly with Emil’ Alexandrovich Mendgeritsky) [14, 22], and on the kinetics of zinc electrodes in alkaline solutions [16-21, 26].
![]() As part of his strategic planning, Bagotsky developed methods of comparing the performance of different power sources. In particular, and long before D. Ragone, he constructed plots of “specific energy” versus “specific power”. Unfortunately he never published his idea. However, he did publish a general monograph entitled “Advances in Chemical Power Sources” ([2] in the Monograph subsection, with Vladimir Nikolaevich Flyorov), which became the standard handbook for Soviet engineers and researchers. This monograph was also published in Polish and Romanian.
As part of his strategic planning, Bagotsky developed methods of comparing the performance of different power sources. In particular, and long before D. Ragone, he constructed plots of “specific energy” versus “specific power”. Unfortunately he never published his idea. However, he did publish a general monograph entitled “Advances in Chemical Power Sources” ([2] in the Monograph subsection, with Vladimir Nikolaevich Flyorov), which became the standard handbook for Soviet engineers and researchers. This monograph was also published in Polish and Romanian.
![]() Inside the Soviet Union, two important organizational changes occurred at the end of the nineteen fifties. First, the Institute of Electrochemistry of the Academy of Sciences was founded in 1958, with Academician A. N. Frumkin at its head. Second, a large fuel cell research program was started. From the outset, Frumkin did his best to involve Bagotsky in the new venture. Even though he was still formally part of the VNIIT research staff, Bagotsky worked very closely with the Institute of Electrochemistry, and in 1965 he moved there permanently. Initially he operated as a Head of Laboratory, but later he became a Head of Department.
Inside the Soviet Union, two important organizational changes occurred at the end of the nineteen fifties. First, the Institute of Electrochemistry of the Academy of Sciences was founded in 1958, with Academician A. N. Frumkin at its head. Second, a large fuel cell research program was started. From the outset, Frumkin did his best to involve Bagotsky in the new venture. Even though he was still formally part of the VNIIT research staff, Bagotsky worked very closely with the Institute of Electrochemistry, and in 1965 he moved there permanently. Initially he operated as a Head of Laboratory, but later he became a Head of Department.
![]() From 1960 onwards, Bagotsky became one of the leading researchers in the Soviet Fuel Cell program. He became Vice-chairman of the Scientific Council on Fuel Cells of the Academy of Sciences of the USSR, and simultaneously the Vice-chairman of the Interdepartmental Committee on the Development of Electric Vehicles. From the outset, Bagotsky had grasped the importance of porous electrodes. In 1963 he carried out work with Iosif Grigor’evich Gurevich on the study of flooded porous electrodes under various experimental conditions [27-29, 35, 46]. Later, this work was continued with Yurii Mironovich Volfkovich [63, 76, 77, 83, 86, 88, 93, 98, 99, 108, 109, 173], and the results were eventually compiled in a monograph [3]. The development of flooded porous electrodes also necessitated the development of improved porosimetry techniques. Bagotsky did much to popularize the method of Standard Contact Porosimetry, invented in collaboration with Yu. M. Vol’fkovich, [224, 225, 237, 272, 294]. This technique has several advantages over traditional mercury porosimetry, and allows the investigation of the structure and properties of all kinds of porous materials, including frail solids and powders. The method is relatively simple, non-destructive, and is not connected with the use of mercury.
From 1960 onwards, Bagotsky became one of the leading researchers in the Soviet Fuel Cell program. He became Vice-chairman of the Scientific Council on Fuel Cells of the Academy of Sciences of the USSR, and simultaneously the Vice-chairman of the Interdepartmental Committee on the Development of Electric Vehicles. From the outset, Bagotsky had grasped the importance of porous electrodes. In 1963 he carried out work with Iosif Grigor’evich Gurevich on the study of flooded porous electrodes under various experimental conditions [27-29, 35, 46]. Later, this work was continued with Yurii Mironovich Volfkovich [63, 76, 77, 83, 86, 88, 93, 98, 99, 108, 109, 173], and the results were eventually compiled in a monograph [3]. The development of flooded porous electrodes also necessitated the development of improved porosimetry techniques. Bagotsky did much to popularize the method of Standard Contact Porosimetry, invented in collaboration with Yu. M. Vol’fkovich, [224, 225, 237, 272, 294]. This technique has several advantages over traditional mercury porosimetry, and allows the investigation of the structure and properties of all kinds of porous materials, including frail solids and powders. The method is relatively simple, non-destructive, and is not connected with the use of mercury.
![]() Although the study of flooded porous electrodes was a dominant theme at that stage of his careeer, Bagotsky was by no means limited to that topic. He was always fully aware of the importance of gas diffusion electrodes, and especially their application to fuel cells. Numerous studies of gas diffusion electrodes were completed under Bagotsky’s auspices in the nineteen seventies [128, 140, 162, 168, 180, 187, 189, 190, 213, 214, 226, 248]. Unsurprisingly, many of these studies were aimed at understanding the relation between the structure of porous materials and their electrochemical peformance. Of special interest in this regard are the papers [73, 74, 126]. In these publications it is possible to see Bagotsky building on Frumkin’s idea of replacing the complex geometry of real pores with a simple one dimensional model.
Although the study of flooded porous electrodes was a dominant theme at that stage of his careeer, Bagotsky was by no means limited to that topic. He was always fully aware of the importance of gas diffusion electrodes, and especially their application to fuel cells. Numerous studies of gas diffusion electrodes were completed under Bagotsky’s auspices in the nineteen seventies [128, 140, 162, 168, 180, 187, 189, 190, 213, 214, 226, 248]. Unsurprisingly, many of these studies were aimed at understanding the relation between the structure of porous materials and their electrochemical peformance. Of special interest in this regard are the papers [73, 74, 126]. In these publications it is possible to see Bagotsky building on Frumkin’s idea of replacing the complex geometry of real pores with a simple one dimensional model.
![]() The industrial need for knowledge about porous electrodes was self-evident; but, in the development of fuel cells, another major requirement was knowledge of electrocatalysis. Bagotsky’s interest in electrocatalysis covered both the general principles of the subject as well as the analysis of special cases. Regarding the general principles, Bagotsky realized how important it was to carry out systematic studies of structural effects, such as particle size, crystallographic orientation, and chemical identity of the support. A Short Letter [78] describing the difference in electrocatalytic activity between smooth and platinized platinum was a milestone. (These classic experiments were carried out by two of Bagotsky’s pupils, Yurii Borisovich Vassiliev and Olga Alekseevna Khazova, and both subsequently remained his co-workers for half a century.) The same topic was also addressed in refs [133, 153, 199, 326], and the difference in behavior between smooth and highly dispersed platinum was investigated with regard to chemisorption, methanol oxidation, and hydrogen ionization. Differences in electrocatalytic behavior were also found between smooth and ruthenized ruthenium [217], and between rhodium and rhodium-plated surfaces [148].
The industrial need for knowledge about porous electrodes was self-evident; but, in the development of fuel cells, another major requirement was knowledge of electrocatalysis. Bagotsky’s interest in electrocatalysis covered both the general principles of the subject as well as the analysis of special cases. Regarding the general principles, Bagotsky realized how important it was to carry out systematic studies of structural effects, such as particle size, crystallographic orientation, and chemical identity of the support. A Short Letter [78] describing the difference in electrocatalytic activity between smooth and platinized platinum was a milestone. (These classic experiments were carried out by two of Bagotsky’s pupils, Yurii Borisovich Vassiliev and Olga Alekseevna Khazova, and both subsequently remained his co-workers for half a century.) The same topic was also addressed in refs [133, 153, 199, 326], and the difference in behavior between smooth and highly dispersed platinum was investigated with regard to chemisorption, methanol oxidation, and hydrogen ionization. Differences in electrocatalytic behavior were also found between smooth and ruthenized ruthenium [217], and between rhodium and rhodium-plated surfaces [148].
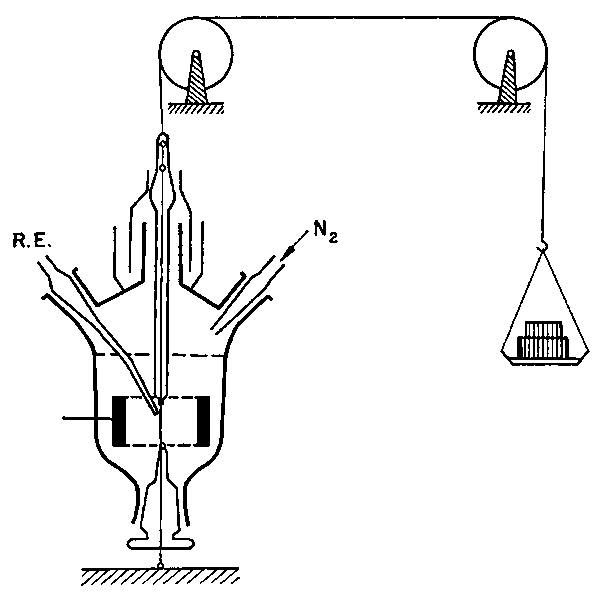 |
| A sketch drawn byV. S. Bagotsky: the cell for electrochemical measurements on a wire under mechanical load (stretching) |
![]() It was inside Bagotsky’s Lab that various pioneering studies of single crystal surfaces of platinum group metals were first attempted [117, 119, 121, 124, 149, 167]. Despite the poor quality of some of the single crystal surfaces used in those works, important lessons were learned about the profound role played by crytallographic orientation. Another highly innovative series of studies related to the role of surface defects [112-114, 134, 149]. These were created mainly by mechanical action (such as surface hardening, stretching, and twisting) although sometimes by neutron irradiation. Surprisingly, it was found that the number density of surface defects had little effect on hydrogen adsorption, methanol adsorption, or oxygen evolution.
It was inside Bagotsky’s Lab that various pioneering studies of single crystal surfaces of platinum group metals were first attempted [117, 119, 121, 124, 149, 167]. Despite the poor quality of some of the single crystal surfaces used in those works, important lessons were learned about the profound role played by crytallographic orientation. Another highly innovative series of studies related to the role of surface defects [112-114, 134, 149]. These were created mainly by mechanical action (such as surface hardening, stretching, and twisting) although sometimes by neutron irradiation. Surprisingly, it was found that the number density of surface defects had little effect on hydrogen adsorption, methanol adsorption, or oxygen evolution.
![]() On the other hand, many interesting and important effects in electrocatalysis were discovered for supported electrocatalysts, especially in relation to the nature of the catalyst support. Today, it is widely understood that at least two different effects are involved, namely a particle-size effect and a catalyst-support interaction. Although dozens of Bagotsky’s papers were devoted to the catalyst-support interaction, it remains a subject of intensive research. In a well-known Letter to “Electrokhimiya” [110] Bagotsky and his co-workers reported an increase of electrocatalytic activity of platinum microcrystals when they were deposited on a seemingly inert support (pyrolytic carbon), the size of the effect being inversely related to the size of the crystals. Subsequently this topic was investigated in detail [122, 136, 142, 152, 157, 171, 175, 208, 215, 301, 310, 316, 317, 319, 320, 329]. In order to obtain quantitative measurements of catalyst-support interactions, electron photoemission was also explored [301, 310, 320]. This latter research was implemented by Bagotsky’s pupil, Alexander Matveevich Skundin.
On the other hand, many interesting and important effects in electrocatalysis were discovered for supported electrocatalysts, especially in relation to the nature of the catalyst support. Today, it is widely understood that at least two different effects are involved, namely a particle-size effect and a catalyst-support interaction. Although dozens of Bagotsky’s papers were devoted to the catalyst-support interaction, it remains a subject of intensive research. In a well-known Letter to “Electrokhimiya” [110] Bagotsky and his co-workers reported an increase of electrocatalytic activity of platinum microcrystals when they were deposited on a seemingly inert support (pyrolytic carbon), the size of the effect being inversely related to the size of the crystals. Subsequently this topic was investigated in detail [122, 136, 142, 152, 157, 171, 175, 208, 215, 301, 310, 316, 317, 319, 320, 329]. In order to obtain quantitative measurements of catalyst-support interactions, electron photoemission was also explored [301, 310, 320]. This latter research was implemented by Bagotsky’s pupil, Alexander Matveevich Skundin.
![]() Among many electrochemical reactions, two were of special interest for Bagotsky throughout his scientific carrier, namely, cathodic reduction of oxygen and anodic oxidation of small organic molecules. Both processes are vital to fuel cell operation. His early work on oxygen reduction on mercury [8, 10-12] was extended in the nineteen sixties and seventies to platinum group metals [47, 129, 135, 141, 151, 153, 158, 188, 196, 203, 216, 219, 247, 263, 269, 278, 290, 291], silver [94, 143, 153, 156, 166, 205, 207, 212, 232, 234, 244], carbon [284, 296, 314], nickel [72, 80, 95, 97, 101, 104, 132, 163, 177, 193, 195, 204-206, 231, 257], as well as supported platinum [197, 252, 281, 332] and supported silver [236, 253]. In addition to numerous detailed studies of oxygen adsorption and reduction, Bagotsky also carried out a number of theoretical calculations of kinetic parameters [92, 107, 150, 282].
Among many electrochemical reactions, two were of special interest for Bagotsky throughout his scientific carrier, namely, cathodic reduction of oxygen and anodic oxidation of small organic molecules. Both processes are vital to fuel cell operation. His early work on oxygen reduction on mercury [8, 10-12] was extended in the nineteen sixties and seventies to platinum group metals [47, 129, 135, 141, 151, 153, 158, 188, 196, 203, 216, 219, 247, 263, 269, 278, 290, 291], silver [94, 143, 153, 156, 166, 205, 207, 212, 232, 234, 244], carbon [284, 296, 314], nickel [72, 80, 95, 97, 101, 104, 132, 163, 177, 193, 195, 204-206, 231, 257], as well as supported platinum [197, 252, 281, 332] and supported silver [236, 253]. In addition to numerous detailed studies of oxygen adsorption and reduction, Bagotsky also carried out a number of theoretical calculations of kinetic parameters [92, 107, 150, 282].
![]() One result of Bagotsky’s broad approach to electrooxidation of organic compounds was the development of a generalized reaction scheme. This involved the dissociative chemisorption of organic species, the simultaneous chemisorption of oxygen-containing species, and finally the reaction between the chemisorbed species to create oxidized products. The first papers of this kind appeared in 1963 [24, 25]. In an early review [34], Bagotsky and Yurii Borisovich Vassiliev considered the anodic oxidation of a large number of low-molecular weight substances (formic acid, oxalic acid, formaldehyde, glycerol, butanol, glucose…) all within one framework. Later, this general approach was extended to methanol adsorption and oxidation on platinum [36, 40, 43, 45, 48, 49, 51-53, 57, 62, 66, 84, 85, 100], as well as on other platinum group metals: [44] (palladium), [89, 91, 105, 106, 115, 120, 148] (iridium), [148] (rhodium), [217, 256] (ruthenium). Electrooxidation of ethylene glycol [54, 55], formic acid [68, 70, 79, 87, 90], and formaldehyde [293] were also studied. General summaries can be found in refs [240, 275]. A similar approach to the cathodic reduction of simple organic substances was developed in refs [288, 289, 297, 298].
One result of Bagotsky’s broad approach to electrooxidation of organic compounds was the development of a generalized reaction scheme. This involved the dissociative chemisorption of organic species, the simultaneous chemisorption of oxygen-containing species, and finally the reaction between the chemisorbed species to create oxidized products. The first papers of this kind appeared in 1963 [24, 25]. In an early review [34], Bagotsky and Yurii Borisovich Vassiliev considered the anodic oxidation of a large number of low-molecular weight substances (formic acid, oxalic acid, formaldehyde, glycerol, butanol, glucose…) all within one framework. Later, this general approach was extended to methanol adsorption and oxidation on platinum [36, 40, 43, 45, 48, 49, 51-53, 57, 62, 66, 84, 85, 100], as well as on other platinum group metals: [44] (palladium), [89, 91, 105, 106, 115, 120, 148] (iridium), [148] (rhodium), [217, 256] (ruthenium). Electrooxidation of ethylene glycol [54, 55], formic acid [68, 70, 79, 87, 90], and formaldehyde [293] were also studied. General summaries can be found in refs [240, 275]. A similar approach to the cathodic reduction of simple organic substances was developed in refs [288, 289, 297, 298].
 |
| Vladimir Sergeevich during IELAN period and in his former IELAN office during a visit to Moscow from USA (in color) |
![]() Being a world expert on the oxygen reduction reaction, Bagotsky followed developments with keen interest. He was particularly fascinated by the use of macrocyclic compounds as molecular catalysts for oxygen reduction. Although the electrocatalytic activity of some metal phthalocyanines and some substituted porphyrins had been known since the nineteen seventies, their long-term stability was questionable, and so Bagotsky carried out a series of experiments to test their thermal response. The results were published in refs [273, 276]. The influence of cobalt phthalocyanine, tetraphenylporphyrin, and tetrabenzoporphyrin increased noticeably after heat-treatment. Of course, heat treatment normally results in destruction of organic compounds, so it was a great surprise when the electrodes retained their high activity. Bagotsky’s lecture at the 3rd International Symposium “Elektrochemische Stromquellen” in Dresden (1978) created a furore!
Being a world expert on the oxygen reduction reaction, Bagotsky followed developments with keen interest. He was particularly fascinated by the use of macrocyclic compounds as molecular catalysts for oxygen reduction. Although the electrocatalytic activity of some metal phthalocyanines and some substituted porphyrins had been known since the nineteen seventies, their long-term stability was questionable, and so Bagotsky carried out a series of experiments to test their thermal response. The results were published in refs [273, 276]. The influence of cobalt phthalocyanine, tetraphenylporphyrin, and tetrabenzoporphyrin increased noticeably after heat-treatment. Of course, heat treatment normally results in destruction of organic compounds, so it was a great surprise when the electrodes retained their high activity. Bagotsky’s lecture at the 3rd International Symposium “Elektrochemische Stromquellen” in Dresden (1978) created a furore!
![]() At the beginning of the nineteen seventies, the attention of electrochemists all over the world was drawn to lithium batteries. The successful commercialization of such devices (both primary and secondary) by various Japanese companies prompted a global shift in research from fuel cells to lithium-based systems. The Institute of Electrochemistry in Moscow could not ignore these developments, and a special lithium group was convened in Bagotsky’s Department, headed by Yurii Mikhailovich Povarov. From the outset the interests of this group were focused on the most powerful primary system, namely lithium-thionyl chloride. It must be remembered that experimental work on this system was very difficult due to the high reactivity of lithium metal, and the fact that the solvent could react violently with water. Nevertheless, Bagotsky’s team made significant progress in elucidating the mechanism of thionyl chloride reduction [271, 280]. They also explored the behaviour of porous cathodes [330, 334, 11 in Book Chapter sub-section], and the mechanism of passivation of lithium [327, 338, 340]. Later the same group also studied the effect of various macrocyclic compounds, including porphyrin derivatives, on the cathodic half-cell reaction [331, 333, 335, and 337]. A wide variety of carbon electrode materials was also studied, including carbon black, activated carbon, and graphitized cloth [334]. It was found that the pores in the carbon electrodes filled up non-synchronously, with the large pores filling with lithium chloride first, and the small pores filling second. Evidently, the pore size distribution was an important factor in the battery performance.
At the beginning of the nineteen seventies, the attention of electrochemists all over the world was drawn to lithium batteries. The successful commercialization of such devices (both primary and secondary) by various Japanese companies prompted a global shift in research from fuel cells to lithium-based systems. The Institute of Electrochemistry in Moscow could not ignore these developments, and a special lithium group was convened in Bagotsky’s Department, headed by Yurii Mikhailovich Povarov. From the outset the interests of this group were focused on the most powerful primary system, namely lithium-thionyl chloride. It must be remembered that experimental work on this system was very difficult due to the high reactivity of lithium metal, and the fact that the solvent could react violently with water. Nevertheless, Bagotsky’s team made significant progress in elucidating the mechanism of thionyl chloride reduction [271, 280]. They also explored the behaviour of porous cathodes [330, 334, 11 in Book Chapter sub-section], and the mechanism of passivation of lithium [327, 338, 340]. Later the same group also studied the effect of various macrocyclic compounds, including porphyrin derivatives, on the cathodic half-cell reaction [331, 333, 335, and 337]. A wide variety of carbon electrode materials was also studied, including carbon black, activated carbon, and graphitized cloth [334]. It was found that the pores in the carbon electrodes filled up non-synchronously, with the large pores filling with lithium chloride first, and the small pores filling second. Evidently, the pore size distribution was an important factor in the battery performance.
![]() As for the passive films on the lithium surface, it was found that they exhibit self-healing properties. They have an inner layer which is thin and poorly crystalline, and an outer layer which is thick and crystalline. Overall, the film exhibits p-type semiconductivity, with a small hole concentration of circa 1014 cm-3. Nevertheless, it is sufficient to allow the slow growth of the secondary film. Because of various reasons, in particular the secrecy of early lithium batteries R&D, these and some other important results remained unpublished in scientific journals.
As for the passive films on the lithium surface, it was found that they exhibit self-healing properties. They have an inner layer which is thin and poorly crystalline, and an outer layer which is thick and crystalline. Overall, the film exhibits p-type semiconductivity, with a small hole concentration of circa 1014 cm-3. Nevertheless, it is sufficient to allow the slow growth of the secondary film. Because of various reasons, in particular the secrecy of early lithium batteries R&D, these and some other important results remained unpublished in scientific journals.
![]() In his retirement, Bagotsky maintained close contact with his former colleagues in Moscow even after moving to the USA. A lot of people from the former Bagotsky’s Depertment are still working in the Frumkin Institute, forming the Laboratory of the Processes in Power Sources. Amazing all ot us, he continued to work on textbooks and monographs well past his 90th birthday; a special session of the American Electrochemical Society was arranged in his honour as a part of the 218th meeting of the Electrochemical Society in October 2010. His passing is a great loss.
In his retirement, Bagotsky maintained close contact with his former colleagues in Moscow even after moving to the USA. A lot of people from the former Bagotsky’s Depertment are still working in the Frumkin Institute, forming the Laboratory of the Processes in Power Sources. Amazing all ot us, he continued to work on textbooks and monographs well past his 90th birthday; a special session of the American Electrochemical Society was arranged in his honour as a part of the 218th meeting of the Electrochemical Society in October 2010. His passing is a great loss.
 |
| Celebration of Bagotsky’s 80-year jubilee in his Moscow flat. From left to right: Nina Vladimirovna Osetrova, Alexander Matveevich Skundin, Evgeniya Ivanovna Khrushcheva, Irina Evgenyevna Yablokova, Vladimir Sergeevich Bagotsky |
![]() The authors are grateful to Professor Steven Fletcher for his kind help with preparation of this text, and to Dr Natalya V. Bagotskaya, who provided the photos and other original materials.
The authors are grateful to Professor Steven Fletcher for his kind help with preparation of this text, and to Dr Natalya V. Bagotskaya, who provided the photos and other original materials.
Alexander M. Skundin, Galina A. Tsirlina
|