Ion transfer processes manifest themselves in ion intercalation, amalgamation and electrodeposition reactions. These reactions are undoubtedly more complex for the theoretical description, as the activation process in this case involves both the change in the solvent coordinate and the coordinate of the ion. In the most general case, if ion transfer is accompanied by the ET event, it is also not clear which stage is the slowest (and the rate limiting) – ion or electron transfer. Metal ion intercalation into oxide and carbon matrices presents an opportunity to examine the kinetic patterns of slow ion transfer, as for these reactions a pronounced solvent dependence is observed, which is unlikely to be associated with slow solid-state ET. Slow ion transfer step is supposed to determine the rates of charge transfer across the solvent/electrolyte interface for a wide range of intercalating ions and hosts.
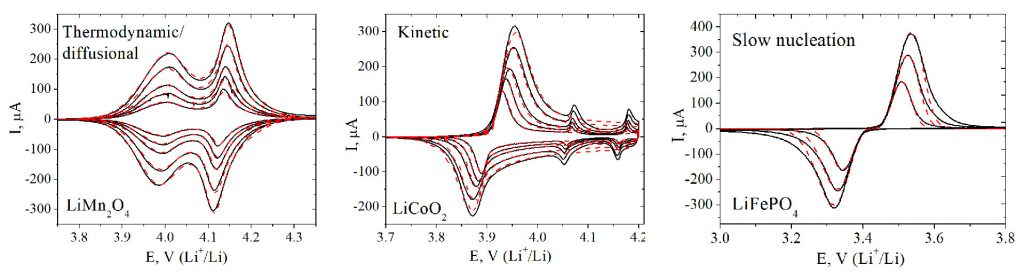
One of the research directions focuses on the elucidation of the intercalation process limiting step under different experimental conditions (solid state diffusion of the ion in the host, charge transfer across the electrode/electrolyte interface, nucleation of a new phase, chemical step of ion desolvation, etc.) by means of voltammetric, chronoamperometric and impedance responses of the model systems. The obtained data allow for specifying model systems, for which the precise determination of ion transfer rate constants is possible.
Cyclic voltammograms for a range of model intercalation materials, which exhibit different rate limiting steps (slow diffusion of the ion, slow charge transfer step, slow nucleation of the new phase). S.Yu. Vassiliev et al., Electrochim. Acta. 190 (2016) 1087.
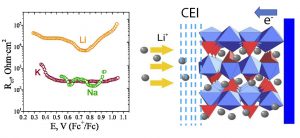
Another research direction concerns the elucidation of the effects of ionic size and charge, solvent properties and reaction layer structure on the ion transfer rates. For organic solvents (e.g. typical metal-ion battery ethylene carbonate and propylene carbonate-based electrolytes) the complicating factor consists in the formation of SEI or CEI layers at the host particles’ surfaces. This problem can be overcome by creating artificial surface layer of known composition and structure. Potential of mean force profiles and reaction layer structure are specified by means of molecular dynamics modeling for the selected systems.
The dependence of the ion transfer rates (Li+, Na+, K+ intercalation into VPO4F host) on the nature of the cation; schematic representation of the metal ion transfer across the material/electrolyte interface. (V.A. Nikitina et al., J. Electrochem. Soc. 164 (2017) A6373).