![]() The behavior of low-temperature hydrogen sensors based on the PbO2/heteropoly compound/Pt electrochemical cells, where heteropoly compound is H4SiW12O40*nH2O or MeH3-xPW12O40*nH2O (Me = Li, Na, K, Rb, NH4, x=0,2,3), in H2-Ar and H2-air atmospheres was studied. All heteropoly compounds studied were shown to be electrocatalytically active in reactions involving hydrogen. The dependences of the emf of the sensors with different electrolytes on the hydrogen concentration (calculated relatively to the standard hydrogen potential in inert media or to the air potential in oxygen-containing media) are identical for all the electrolytes used. The rate of emf relaxation of the sensors upon the impulse admission of hydrogen depends, on the contrary, on both the anion nature (increases in the series from compounds of 12-silicotungstic acid to 12-phosphotungstic acid compounds) and the cation nature, increasing with an increase in the cation size in the series of alkaline metal cations.
The behavior of low-temperature hydrogen sensors based on the PbO2/heteropoly compound/Pt electrochemical cells, where heteropoly compound is H4SiW12O40*nH2O or MeH3-xPW12O40*nH2O (Me = Li, Na, K, Rb, NH4, x=0,2,3), in H2-Ar and H2-air atmospheres was studied. All heteropoly compounds studied were shown to be electrocatalytically active in reactions involving hydrogen. The dependences of the emf of the sensors with different electrolytes on the hydrogen concentration (calculated relatively to the standard hydrogen potential in inert media or to the air potential in oxygen-containing media) are identical for all the electrolytes used. The rate of emf relaxation of the sensors upon the impulse admission of hydrogen depends, on the contrary, on both the anion nature (increases in the series from compounds of 12-silicotungstic acid to 12-phosphotungstic acid compounds) and the cation nature, increasing with an increase in the cation size in the series of alkaline metal cations.
![]() Based on our studies, we proposed the electrochemical solid-state sensor, which can determine hydrogen concentrations from 10-2 to 5 vol.% in inert gases and air without maintaining constant temperature and hydrostatic regimes in the temperature interval from -60 to +60oC.
Based on our studies, we proposed the electrochemical solid-state sensor, which can determine hydrogen concentrations from 10-2 to 5 vol.% in inert gases and air without maintaining constant temperature and hydrostatic regimes in the temperature interval from -60 to +60oC.
![]() Keywords: hydrogen sensors, heteropolycompounds, potential relaxation, solid electrolytes.
Keywords: hydrogen sensors, heteropolycompounds, potential relaxation, solid electrolytes.
![]() Electrochemical cells using solid electrolytes (superionics) are usually used for hydrogen detection under standard conditions [1-14]. The detection can often be carried out without heating and maintaining constant temperature and hydrostatic regimes. Antimonic acid, Nafion, other organic and hybrid polymers, hydrated Nasicon, heteropoly compounds, and many other superprotonics are used as H+-SE in these cells. The Ag/Ag+ electrodes (Ag, Ag2SO4; Ag, AgCl); transition metal hydrides, for example, TiH2; hydrated oxides, for example, NiO, PbO2; were proposed as RE. Analysis in [4] has shown that the system
Electrochemical cells using solid electrolytes (superionics) are usually used for hydrogen detection under standard conditions [1-14]. The detection can often be carried out without heating and maintaining constant temperature and hydrostatic regimes. Antimonic acid, Nafion, other organic and hybrid polymers, hydrated Nasicon, heteropoly compounds, and many other superprotonics are used as H+-SE in these cells. The Ag/Ag+ electrodes (Ag, Ag2SO4; Ag, AgCl); transition metal hydrides, for example, TiH2; hydrated oxides, for example, NiO, PbO2; were proposed as RE. Analysis in [4] has shown that the system
| PbO2/(NH4)2HPW12O40*nH2O/Pt, O2,H2 | (1) |
gives a fast and high-sensitive response to a change in the hydrogen content in both an inert gas and oxygen-containing media.
![]() The purpose of this work is to study the influence of the composition of the solid electrolytes based on heteropoly compounds on the characteristics of potentiometric H2 sensors similar to (1).
The purpose of this work is to study the influence of the composition of the solid electrolytes based on heteropoly compounds on the characteristics of potentiometric H2 sensors similar to (1).
![]() Phosphotungstic (H3PW12O40*nH2O) and silicotungstic (H4SiW12O40*nH2O) acids, M3PW12O40*nH2O and MH2PW12O40*nH2O salts, where M = H+, (NH4+), Li+, K+, Na+, and Cs+, were used as starting substances. The dependence of the emf of the sensors on the hydrogen concentration in inert (Ar) and oxygen-containing (air) media and emf relaxation of the sensors upon an impulse change in the hydrogen concentration in the medium under consideration were studied.
Phosphotungstic (H3PW12O40*nH2O) and silicotungstic (H4SiW12O40*nH2O) acids, M3PW12O40*nH2O and MH2PW12O40*nH2O salts, where M = H+, (NH4+), Li+, K+, Na+, and Cs+, were used as starting substances. The dependence of the emf of the sensors on the hydrogen concentration in inert (Ar) and oxygen-containing (air) media and emf relaxation of the sensors upon an impulse change in the hydrogen concentration in the medium under consideration were studied.
![]() The cell
The cell
| PbO2/H+-SE/Pt, H2,air or Ar | (2) |
was used in the work, where PbO2 is a reference electrode, and the Pt sponge is a sensitive electrode, whose design is presented in Fig. 1. The sensor was prepared by molding of two electrodes onto the solid electrolyte followed by storage of the cell above a constant humidity of ~50 rel.%. The prepared pellet was placed in a cylindrical shell of an insulating material in a titanium body. Measurements detected the potential difference between the titanium body, which was in direct contact with the platinum electrode, and a titanium current contact jaw connecting with the reference electrode (titanium dioxide).
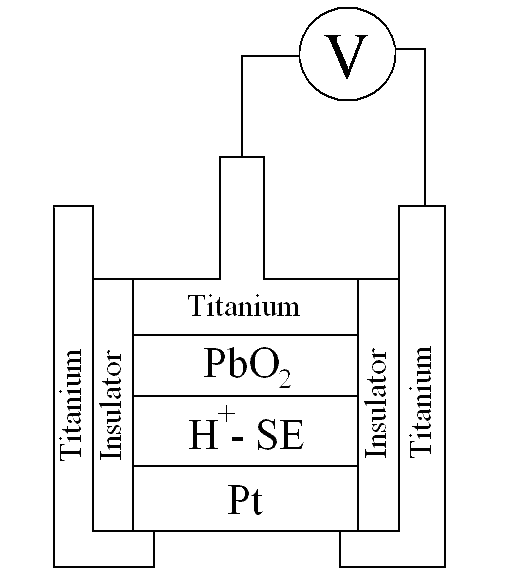 |
| Fig. 1. Design of the electrochemical cell used as a hydrogen sensor. |
![]() The plots of the emf of sensors (2) with heteropoly acids as solid electrolytes vs. hydrogen concentration in air-less and air atmospheres are presented in Fig. 2. In the absence of oxygen at 20°Ñ, the concentration dependence of the emf for cell (2) with phosphotungstic acid is described by the Nernst equation
The plots of the emf of sensors (2) with heteropoly acids as solid electrolytes vs. hydrogen concentration in air-less and air atmospheres are presented in Fig. 2. In the absence of oxygen at 20°Ñ, the concentration dependence of the emf for cell (2) with phosphotungstic acid is described by the Nernst equation
| E = (1.545±0.002) - (0.028±0.002)log[H2] | (3) |
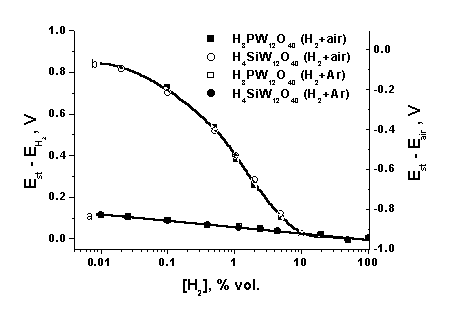 |
| Fig.2. Plots of the potentials of the platinum electrodes in cells (2) with H4SiW12O40*nH2O and H3PW12O40*nH2O vs. hydrogen concentration in inert (a) and oxygen-containing (b) media recalculated to the standard hydrogen potential (left scale) and air potential (right scale). t = 25°C, relative humidity = 52 %. |
![]() These values correspond to hydrogen oxidation at the working electrode
These values correspond to hydrogen oxidation at the working electrode
| H2 - 2e- --> 2H+ | (4) |
![]() Simultaneously, lead dioxide is reduced at the reference electrode
Simultaneously, lead dioxide is reduced at the reference electrode
| PbO2 + 4H+ + 2e- --> Pb2+ + 2H2O | (5) |
![]() The oxygen and hydrogen content in the analyzed medium has no effect on the potential of this electrode (1.4-1.6 V) but it depends on the nature of the solid electrolyte, because the proton activity differs in electrolytes different in nature. The concentration dependence of the emf recalculated to the standard hydrogen electrode (by subtraction of the emf of the sensor at a hydrogen pressure of 1 atm from the measured emf values of the same sensor) are described by the Nernst equation for a two-electron process for all sensors studied (the theoretical value of the pre-logarithmic factor for t = 25°C is 2.303*RT/2F=0.0293). Phosphomolybdic acid turned out to be absolutely inappropriate for operating in the composition of cells (2), because its chemical reduction with molecular hydrogen results in the appearance of a very high fraction of electron conductivity, and the emf of the cell changes irreversibly upon reduction.
The oxygen and hydrogen content in the analyzed medium has no effect on the potential of this electrode (1.4-1.6 V) but it depends on the nature of the solid electrolyte, because the proton activity differs in electrolytes different in nature. The concentration dependence of the emf recalculated to the standard hydrogen electrode (by subtraction of the emf of the sensor at a hydrogen pressure of 1 atm from the measured emf values of the same sensor) are described by the Nernst equation for a two-electron process for all sensors studied (the theoretical value of the pre-logarithmic factor for t = 25°C is 2.303*RT/2F=0.0293). Phosphomolybdic acid turned out to be absolutely inappropriate for operating in the composition of cells (2), because its chemical reduction with molecular hydrogen results in the appearance of a very high fraction of electron conductivity, and the emf of the cell changes irreversibly upon reduction.
![]() In the presence of oxygen, the concentration dependence of the emf is non-Nernst (Fig. 2). Similar concentration plots of the emf are characteristic of several potential-determining processes simultaneously occurring at the electrode. For example, in the case of a hydrogen sensor, we can assume that the stationary potential of the working electrode is established when the currents of the following reactions are equal:
In the presence of oxygen, the concentration dependence of the emf is non-Nernst (Fig. 2). Similar concentration plots of the emf are characteristic of several potential-determining processes simultaneously occurring at the electrode. For example, in the case of a hydrogen sensor, we can assume that the stationary potential of the working electrode is established when the currents of the following reactions are equal:
| H2 --> 2H+ + 2e- | (6) |
| and O2 + 2H+ + 2e- --> H2O2 | (7) |
| or O2 + 4H+ + 4e- --> 2H2O | (8) |
![]() As follows from the theoretical consideration [4], at high hydrogen concentrations the concentration dependence of the emf for cells with different electrolytes approaches the concentration dependence in an oxygen-less atmosphere, indicating that hydrogen ionization is the limiting step in this case. In the medium region of the concentration curve, where the potential is determined by the competition of two processes, viz., hydrogen ionization and oxygen reduction, the concentration dependence is close to linear with the angular coefficient greater than 100 mV/decade. The parameters of the concentration dependence are independent of the anion nature. Recalculation to the air potential was used instead of the recalculation to the standard hydrogen potential for the correct comparison of the results obtained in oxygen-containing media with different electrolytes. For this purpose, the emf of the sensor in air in the absence of hydrogen was subtracted from the current emf of the same sensor (Fig. 2, right).
As follows from the theoretical consideration [4], at high hydrogen concentrations the concentration dependence of the emf for cells with different electrolytes approaches the concentration dependence in an oxygen-less atmosphere, indicating that hydrogen ionization is the limiting step in this case. In the medium region of the concentration curve, where the potential is determined by the competition of two processes, viz., hydrogen ionization and oxygen reduction, the concentration dependence is close to linear with the angular coefficient greater than 100 mV/decade. The parameters of the concentration dependence are independent of the anion nature. Recalculation to the air potential was used instead of the recalculation to the standard hydrogen potential for the correct comparison of the results obtained in oxygen-containing media with different electrolytes. For this purpose, the emf of the sensor in air in the absence of hydrogen was subtracted from the current emf of the same sensor (Fig. 2, right).
![]() The relaxation curves of the emf of the sensor for the impulse hydrogen admission are presented in Fig. 3. However, it is difficult to compare the rates in these coordinates. Therefore, hereinafter we used the transform of the current emf value into the relative values
The relaxation curves of the emf of the sensor for the impulse hydrogen admission are presented in Fig. 3. However, it is difficult to compare the rates in these coordinates. Therefore, hereinafter we used the transform of the current emf value into the relative values
| E = (Et - E0)/(Est - E0) | (9) |
where E0 is the emf of the sensor in the analyzed medium (air or argon) in the absence of hydrogen, Est is the stationary potential of the sensor at a specific hydrogen concentration, and Et is the current emf value (Fig. 4).
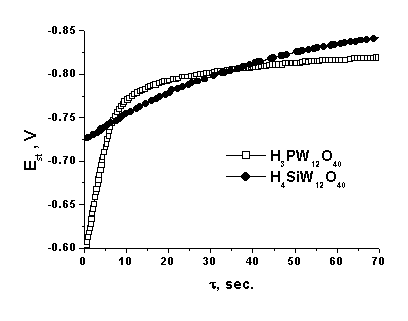 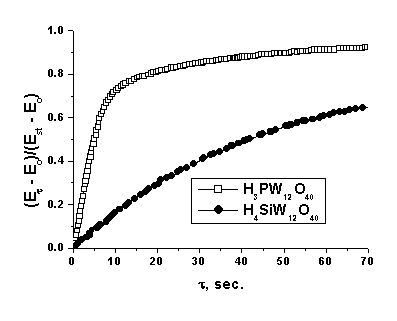 |
| Fig. 3. Relaxation of emf (Est) with an impulse change in the hydrogen concentration for cells (2) with different electrolytes in the absolute (a) and normalized (b) coordinates. t = 25°C, relative humidity = 52 %. In the zero point the hydrogen concentration changes stepwise from 0 to 0.1 vol.%. |
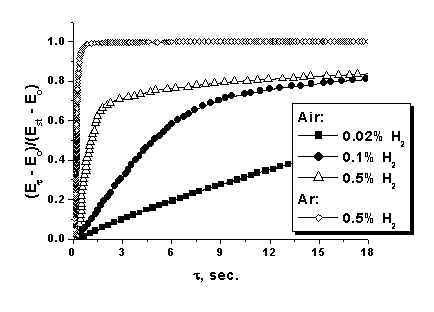 |
| Fig. 4. Relaxation of emf with an impulse change in the hydrogen concentration normalized according to equation (9) for cells (2) with H3PW12O40*nH2O in various gas media. t = 25°C, relative humidity = 52 %. |
![]() The rate of establishment of the standard potentials is rather high, and t90 for the whole studied interval of hydrogen concentrations does not exceed 90 s (0.01%). In the whole studied interval of hydrogen concentrations, the rate of potential relaxation in an inert atmosphere is much higher than that in air and, in addition, depends strongly on the electrolyte nature, which is probably caused by the difference in the rate of the processes at the three-phase electrode/electrolyte/gas interfaces. Compounds of phosphotungstic acid turned out to be most active in these reactions. Thus, although silicotungstic acid is very stable toward chemical reduction by hydrogen and its electron component of conductivity is very low, the rate of relaxation of its potential at the boundary with platinum is much lower than that for phosphotungstic acid.
The rate of establishment of the standard potentials is rather high, and t90 for the whole studied interval of hydrogen concentrations does not exceed 90 s (0.01%). In the whole studied interval of hydrogen concentrations, the rate of potential relaxation in an inert atmosphere is much higher than that in air and, in addition, depends strongly on the electrolyte nature, which is probably caused by the difference in the rate of the processes at the three-phase electrode/electrolyte/gas interfaces. Compounds of phosphotungstic acid turned out to be most active in these reactions. Thus, although silicotungstic acid is very stable toward chemical reduction by hydrogen and its electron component of conductivity is very low, the rate of relaxation of its potential at the boundary with platinum is much lower than that for phosphotungstic acid.
![]() Phosphomolybdic acid is prone to chemical reduction, resulting in a high electron component of conductivity, which leads to the fast degradation of the sensor and poor reproducibility of results of measurements. Phosphotungstic acid in a hydrogen atmosphere is reduced to a less extent than phosphomolybdic acid, and the rates of emf relaxation upon hydrogen pulses are much higher than those in the case of silicotungstic acid. Therefore, we used salts of this acid in the further work.
Phosphomolybdic acid is prone to chemical reduction, resulting in a high electron component of conductivity, which leads to the fast degradation of the sensor and poor reproducibility of results of measurements. Phosphotungstic acid in a hydrogen atmosphere is reduced to a less extent than phosphomolybdic acid, and the rates of emf relaxation upon hydrogen pulses are much higher than those in the case of silicotungstic acid. Therefore, we used salts of this acid in the further work.
![]() Probably, the catalytic activity is primarily determined by the possibility of the heteropoly anion to enter into redox reactions. In order to check this hypothesis, we studied processes in cells with neutral and disubstituted acidic salts of phosphotungstic acid with alkaline metals.
Probably, the catalytic activity is primarily determined by the possibility of the heteropoly anion to enter into redox reactions. In order to check this hypothesis, we studied processes in cells with neutral and disubstituted acidic salts of phosphotungstic acid with alkaline metals.
![]() Heteropoly acids exhibit a strong dependence of their transport parameters on the humidity. This dependence is much weaker for salts (especially insoluble) of phosphotungstic acid (Table 1).
Heteropoly acids exhibit a strong dependence of their transport parameters on the humidity. This dependence is much weaker for salts (especially insoluble) of phosphotungstic acid (Table 1).
![]() These salts possess high proton conductivity, which is often higher than the conductivity of acids. As shown previously, the conductivity in neutral salts is related to the presence of doped cations in the structure. They are transferred over the proton-hydrate shell of the heteropoly compound, while normal structural protons are involved in ion transfer processes in acidic salts.
These salts possess high proton conductivity, which is often higher than the conductivity of acids. As shown previously, the conductivity in neutral salts is related to the presence of doped cations in the structure. They are transferred over the proton-hydrate shell of the heteropoly compound, while normal structural protons are involved in ion transfer processes in acidic salts.
![]() Neutral and acidic disubstituted salts of phosphotungstic acid with monovalent alkaline metal cations and ammonium were chosen as objects for the study. Lithium and sodium salts, as well as the heteropoly acid itself, are well soluble, whereas the solubility of neutral salts of ammonium, potassium, and rubidium is very low.
Neutral and acidic disubstituted salts of phosphotungstic acid with monovalent alkaline metal cations and ammonium were chosen as objects for the study. Lithium and sodium salts, as well as the heteropoly acid itself, are well soluble, whereas the solubility of neutral salts of ammonium, potassium, and rubidium is very low.
![]() The plots of the emf of the sensors with different salts vs. hydrogen concentration are presented in Fig. 5. For all salts, the shape of the concentration dependence is very close to that of the concentration dependence of the acid. After recalculation to the air potential, the dependence also numerically coincides with the concentration dependence of the acid.
The plots of the emf of the sensors with different salts vs. hydrogen concentration are presented in Fig. 5. For all salts, the shape of the concentration dependence is very close to that of the concentration dependence of the acid. After recalculation to the air potential, the dependence also numerically coincides with the concentration dependence of the acid.
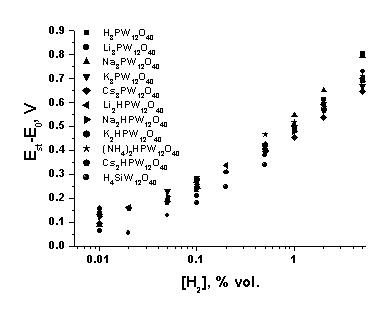 |
| Fig. 5. Concentration plots of the platinum electrode potential recalculated to the air potential for different proton electrolytes in air at 25°C and relative humidity 52 %. |
![]() The concentration plots for different salts are close to each other, while the relaxation rates unambiguously correlate with the cation size, increasing from lithium to cesium (Fig. 6).
The concentration plots for different salts are close to each other, while the relaxation rates unambiguously correlate with the cation size, increasing from lithium to cesium (Fig. 6).
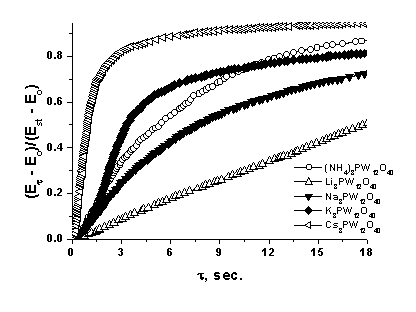 |
| Fig. 6. Relaxation of the emf of the sensors with different electrolytes in air with the stepwise change in the hydrogen concentration from 0 to 1%. |
![]() Ammonium salts of phosphotungstic acid are most stable to temperature oscillations and humidity of the environment. They were used in the further work. However, at low humidity values, the conductivity of the salt and indications of the sensor began to change significantly and, in addition, when the sensor stayed for a long time in an atmosphere with a great hydrogen content, a considerable (although somewhat lower than that in the case of phosphotungstic acid) electron component of conductivity appeared.
Ammonium salts of phosphotungstic acid are most stable to temperature oscillations and humidity of the environment. They were used in the further work. However, at low humidity values, the conductivity of the salt and indications of the sensor began to change significantly and, in addition, when the sensor stayed for a long time in an atmosphere with a great hydrogen content, a considerable (although somewhat lower than that in the case of phosphotungstic acid) electron component of conductivity appeared.
![]() Thus, based on the studies performed, we can choose the optimum design of the hydrogen sensor. We propose the four-layer cell containing two solid electrolytes. Platinum directly contacts with ammonium salt of phosphotungstic acid, and the rate of hydrogen potential relaxation is maximal in this case. Lead dioxide contacts with silicotungstic acid, which is not reduced chemically on the interaction with hydrogen, that is, electron current is absent in the open-loop system.
Thus, based on the studies performed, we can choose the optimum design of the hydrogen sensor. We propose the four-layer cell containing two solid electrolytes. Platinum directly contacts with ammonium salt of phosphotungstic acid, and the rate of hydrogen potential relaxation is maximal in this case. Lead dioxide contacts with silicotungstic acid, which is not reduced chemically on the interaction with hydrogen, that is, electron current is absent in the open-loop system.
![]() The relaxation rate decreases with the temperature decrease, whereas the concentration dependence remains unchanged (Fig. 7).
The relaxation rate decreases with the temperature decrease, whereas the concentration dependence remains unchanged (Fig. 7).
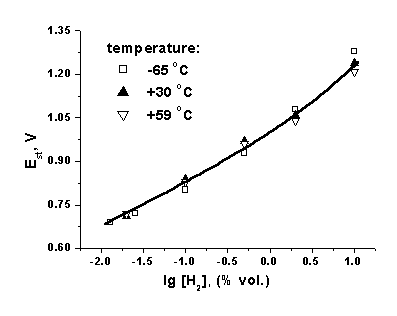 |
| Fig. 7. Concentration plots of the absolute emf values of the sensors in air at different temperatures. |
![]() This work was financially supported by the Russian Foundation for Basic Research (Project # 03-03-32422a).
This work was financially supported by the Russian Foundation for Basic Research (Project # 03-03-32422a).
Table 1. Plots of the amount of water of crystallization, conductivity, and activation energy of heteropolycompounds used in the work on the humidity of the environment
| Heteropolycompound | Íumidity, % | n(H2O) | s273(kOm*cm)-1 | E, eV |
| H3PW12O40 | 84 | 21 | 5.25 | 0.431 |
| 67 | 19 | 3.72 | 0.432 | |
| 57 | 12 | 1.3 | 0.427 | |
| 36 | 7.5 | 0.83 | 0.351 | |
| 16 | 6 | 0.35 | 0.278 | |
| H4SiW12O40 | 36 | 14-22 | 12 | 0.34 |
| 16 | 9 | 1.8 | 0.34 | |
| 5 | 4.6 | 2.14*10-4 | 0.51 | |
| - | 3.3 | 2.15*10-5 | 0.53 | |
| Li3PW12O40 | 95 | 27 | 18.4 | 0.287 |
| 84 | 24 | 2.4 | 0.343 | |
| 16-57 | 13 | 0.35 | 0.403 | |
| Li2HPW12O40 | 16-57 | 13 | 0.84 | - |
| Na3PW12O40 | 94 | 14 | 1.6 | 0.400 |
| 16-84 | 11 | 0.49 | 0.423 | |
| - | 9.5 | 0.24 | 0.455 | |
| - | 6 | 0.081 | 0.420 | |
| - | 2.5 | 2.5*10-3 | 0.480 | |
| Na2HPW12O40 | 94 | 15 | 0.832 | 0.423 |
| 16-84 | 13 | 0.62 | 0.455 | |
| - | 11 | 0.438 | 0.430 | |
| - | 7 | 0.037 | 0.446 | |
| - | 6 | 0.013 | 0.482 | |
| - | 5 | 7.4*10-3 | 0.464 | |
| K3PW12O40 | - | 11 | 17 | - |
| K2HPW12O40 | - | 12 | 26.5 | - |
| Cs3PW12O40 | 94 | 12 | 19.5 | 0.223 |
| 57 | 10 | 9 | 0.224 | |
| 36 | 7.5 | 4.4 | 0.223 | |
| 16 | 6 | 2.1 | 0.264 | |
| - | 5 | 1.1 | 0.248 | |
| Cs2HPW12O40 | 84 | 12 | 42.5 | 0.224 |
| 51 | 11 | 29.4 | 0.224 | |
| (NH4)3PW12O40 | 84 | 10-12 | 10.1 | 0.205 |
| 57 | 10-12 | 10.6 | 0.206 | |
| 36 | 10 | 11.4 | 0.208 | |
| 16 | 6-7 | 6 | 0.213 | |
| (NH4)2HPW12O40 | 84 | 10-12 | 23 | 0.195 |
| 57 | 10-12 | 26 | 0.194 | |
| 36 | 10 | 23 | 0.200 | |
| 16 | 7 | 7.9 | 0.206 |